Keeping this in consideration. Potassium iodide solution chlorine water - potassium chloride Iodine brown neutralisation reaction.
Chemistry Rates Of Reaction Marble Chips And Hydrochloric Acid Gcse Science Marked By Teachers Com
It is used for acid neutralization in the chemical industry as well.
. Often used as a source of calcium carbonate this makes the chemical a perfect fit in any educational or research lab dealing with chemistry or biology. Between marble chips and hydrochloric acid. In the first part marble chips must be added to hydrochloric acid and the volume of gas collected and measured over time.
Equipment used My input variable is the temperature of the hydrochloric acid. 2 A student uses this apparatus to study the rate of the reaction between marble chips and dilute hydrochloric acid. Marble chips White Limestone are mostly made up of calcium carbonate which is an alkaline compound.
This is a high quality chemical product manufactured. Powdered Marble is also used in calcium addictive feeds to dairy animals like chickens and cows. Hope it helps u.
As the marble chips react with the acid carbon. The concentration of hydrochloric acid used. Magnet to pull out the iron nails water to dissolve the salt followed by filtration and evaporate water sieve to separate the marble chips I assume they are relatively large in size compared to sand granules and that leaves the sand behind.
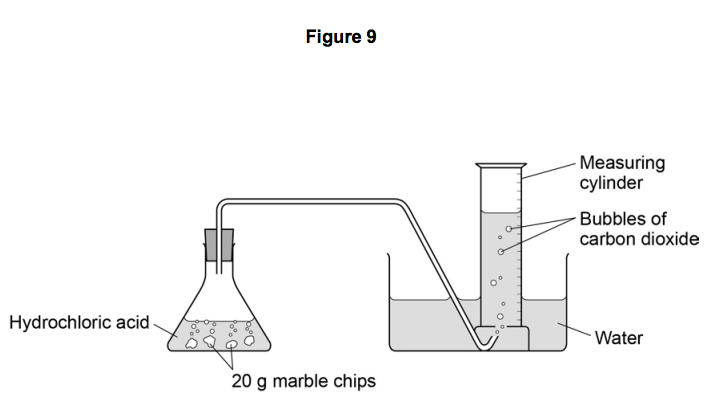
PRODUCT AND COMPANY IDENTIFICATION Product Name. Marble chips calcium carbonate Dilute hydrochloric acid 1M Limewater solution Ca OH 2. This will lead to graphical analysis to calculate rate as well as an appreciation for how the rate may change with varying concentration of acidtemperaturesurface area of marble chips.
Measure 5ml of limewater into the first test tube and set aside in a test tube rack. Marble is used in making sculptures and ornaments. Part 1 - Investigate the rate of a reaction by measuring the production of a gas.
The size of the marble chips. The rate of this reaction can be changed by changing the size of the marble chips. Obtain about 05 g of calcium carbonate marble chips and measure the precise mass.
If the hydrochloric acid isstrongconcentrated it acts upon the marble and corrodes itliberating carbon dioxide. The exact chemical composition of marble will greatly vary dependong on the location and the minerals or impurities present in the limestone during recrystallization. How long does it take to fill the gas syringe in this experiment.
Marble Chips Experiment - 601 Words 123 Help Me Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas. Likewise what are marble chips used for in chemistry. Marble is often crushed and used for acid neutralization in streams lakes and soils.
Calcium chloride and water are also made. Hydrochloric acid to react with the marble chips independent variable Marble chips to react with the acid dependent variable Stopwatch to accurately time the experiment Spatula to handle the marble chips Measuring cylinder to precisely measure out different concentrations of hydryochloric acid Electric balance to measure the mass g of. Innovating Sciences lab-grade calcium carbonate marble chips come in a 500g bottle.
It is used as an inert filler in pills. Address 05 kandi BR. It is used for acid neutralization in the chemical industry as well.
Calcium Carbonate Marble Chips SynonymsGeneric Names. 38-42 Lime CaO 20-25 Silica SiO2 2-4 Alumina Al2O3 15-25 various oxides NaO and MgO and 30-32. Pharmaceutical antacid medicines such as Tums contain calcium carbonate which is sometimes made from powdered marble.
The speed at which CO 2 is produced. Also what are marble chips used for in chemistry. Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
Pharmaceutical antacid medicines such as Tums contain calcium carbonate which is sometimes made from powdered marble. The mass of the marble chips. An investigation of the reaction between marble chips and hydrochloric acid.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas. Jan 6 2012. Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
One may also ask what is the. Chemistry Coursework Rate Of Reaction Hydrochloric Acid And Marble Chips in the form while placing the order provide us with the required Chemistry Coursework Rate Of Reaction Hydrochloric Acid And Marble Chips materials to use in case you have any and proceed with the payment via PayPal. Columbus Chemical Industries Inc.
Add 5 ml of hydrochloric acid to the marble chips and seal with the stopper. Marble is made from limestone under the influence of heat and pressure. Place the marble chips into the other test tube.
Marble chips are calcium carbonate. Each bottle has safe handling and storage procedures printed directly on the bottle. Industrial Manufacturing or Laboratory use Manufacturer.
Marble is often crushed and used for acid neutralization in streams lakes and soils. The amount of hydrochloric acid used. VARIABLES The variables in this experiment are.
Antacid medicines contain CaCo₃ of powdered Marble used to reduce acid reflux in patients. Typically marble is composed of the following major constituents. Calcium chloride solution is also formed.
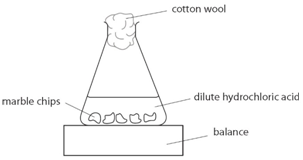
Using the apparatus shown the change in mass of carbon dioxide can be measure with time. Effect of surface area on rates of reaction Answer. Chemistry Coursework Rate Of Reaction Marble Chips Record the mass at time 15 seconds.
GCSEIGCSE Science-Chemistry Quiz on the RATES of. The temperature of the hydrochloric acid. Revised on 06262012 Page 1 of 6 Safety Data Sheet Calcium Carbonate Marble Chips 1.
Marble chips calcium carbonate CaCO 3 react with hydrochloric acid HCl to produce carbon dioxide gas. Marble powder is used in the pharmaceutical industry. The rate of this reaction can be changed by changing the size of the marble chips.

Laboratory Grade Marble Chips Calcium Carbonate 500g The Curated Chemical Collection Amazon Com Industrial Scientific

3 15 Practical Investigate The Effect Of Changing The Surface Area Of Marble Chips And Of Changing The Concentration Of Hydrochloric Acid On The Rate Of Reaction Between Marble Chips And Dilute Hydrochloric
0 Comments